I absolutely love what’s just happened.
Medicine is full of examples where there’s been failure after failure to treat a specific health condition, only for a treatment to appear from a completely different area of research.
Take knee osteoarthritis for example. We haven't had a good treatment option to add to diet and exercise. But then GLP-1 therapies, originally designed to help type 2 diabetics lower their sugar levels, started to show significant benefits for arthritis as well.
Then there’s penicillin. Its discovery was a complete accident, but it went on to have an enormous positive impact on a range of deadly diseases.
These serendipitous moments are amazing, and it looks like the same thing has happened for dementia. Recent research has uncovered a surprising beneficial effect from a vaccine developed to address a problem that, on the surface, appears completely unrelated. But we may have never gotten the data without an unusual circumstance that let researchers run a test no ethics board would ever have allowed.
If the results hold, this could be one of the biggest breakthroughs in decades when it comes to dementia.
Table of Contents
The background story
After massive investments and hundreds of failed trials, we still lack highly effective preventative measures or treatments for dementia [1].

And there’s a lot we don’t know about what causes the condition. But recently, scientists have turned their focus to an unconventional suspect: viruses [2].
For instance, experiments in mice have shown that these viruses stimulate the production of a protein in the brain that helps fight off the infection. But it’s the same protein that clumps together to form the amyloid plaques that are a key feature of Alzheimer’s disease. So the brain’s defense backfires. It fights off the virus, but accelerates processes that contribute to dementia [2].
A chief suspect? The varicella zoster virus — the same virus responsible for chickenpox in childhood. We may hardly remember the infection from when we were young. But the problem is that this virus lurks quietly in our systems, often re-emerging late in life as a painful case of shingles [3].
But even when we don’t notice a pronounced flare-up, as in the case of shingles, the varicella zoster virus can undergo quieter reawakenings we don’t see. Researchers theorize that, in both cases, the virus acts as a chronic stressor to our immune systems. This can drive inflammation in our brain and interfere with its immune functioning [4].
This virus isn’t just linked to brain changes like amyloid plaques that drive Alzheimer’s disease. It also shows signs of contributing to another kind of dementia — vascular dementia. This variety is driven by problems originating in the blood vessels in the brain. We're talking about things like high blood pressure, plaque buildup, and strokes. The varicella zoster virus is linked to the kind of damage usually caused by these factors [4].
So these observations raise an obvious question. Could preventing the flare-up of the varicella zoster virus that causes shingles also help us avoid the damage that appears to accelerate the development of dementia? [4]
Observational studies have pointed to a positive answer.
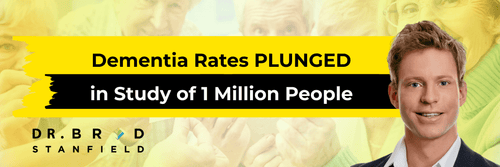
In one example, researchers analyzed health records from two large databases in the U.S. They established two groups at the start of the study period: those who had a shingles vaccination and those who didn’t. Then they checked for new diagnoses of dementia over about 8 years. Participants were at least 65 at the beginning and free of dementia [1].
The association between getting the shingles vaccination and a lower dementia risk was pronounced. The risk reduction was 31–35% [1].
But there’s a significant problem. This is just an observational study. With this kind of study, what’s driving the association is always an open question.
It’s like how autism rates go up with increasing organic food sales. Clearly, they aren’t related.

When we’re looking at two groups — one that got the shingles vaccination and one that didn’t — our attention is focused on that key difference. But what if those who got vaccinated were people who just generally took better care of themselves? Maybe that’s what explains the difference. Even though researchers do their best to account for factors that might distort the results, there are so many things that are just difficult to measure.
And there’s actually some evidence that this isn’t just a hypothetical problem. Some observational studies find that getting vaccinated isn’t just associated with lower dementia rates. It’s also associated with a host of other health outcomes that seem quite unlikely to be related to the vaccine [2]. This kind of result would point toward something like overall health consciousness being the real driver of the positive outcomes seen in the vaccination group.
To really know whether the shingles vaccine is protective against dementia, we need a randomized controlled trial. This is when participants are randomly assigned to get the vaccine or a placebo. That’s the best way to eliminate the impact of other factors like health consciousness.
There’s just one little problem. We can’t ethically run a study like this. The shingles vaccine is the standard recommended care for older adults. That’s because previous studies have already proved that the benefits vastly outweigh the risks compared to a placebo [5].
In one such randomized, placebo-controlled trial involving over 15,000 older adults, the vaccine was 97.2% effective at preventing shingles and showed no increase in serious adverse events [5].
So we can’t assign a group of people not to get the shingles vaccine.
The opportunity
That left us in a position where there was a crucial question we wanted to answer but couldn’t.
But then a group of researchers spotted a once-in-a-lifetime opportunity.

The state healthcare system in Wales made a fateful decision back in 2013. Officials had seen the data on the benefits of vaccination to prevent shingles and wanted to make the vaccine broadly available to the population. But, as always, they had to balance costs and benefits.
They drew a bright red line. Those born on or after September 2, 1933 would be eligible for the vaccine. But those born before that date would never be eligible [2].
This policy gave researchers a rare chance. It closely resembled a randomized controlled trial. Researchers compared two groups: one born just before the cutoff, and one born just after. It’s extremely unlikely there would be any important differences between these two groups — they were essentially the same age [2].
They first confirmed that vaccination rates were different between the two groups. For those born before the cutoff, just 0.01% received the vaccine. For those born after, 47.2% received it [2].
So here’s the crucial question. How did that higher rate of shingles vaccine use impact new cases of dementia?
Those eligible for the vaccine had a 1.3% lower absolute and 8.5% lower relative risk of a new dementia diagnosis over 7 years [2].
But only about half the eligible group actually got the vaccine. So researchers used a statistical technique to estimate the true impact for those who did receive it. For that group, there was a 3.5% absolute and nearly 20% relative reduction in dementia diagnoses [2].
That 3.5% absolute reduction may not sound like much. But in clinical research, that’s a major finding. At a population level, this would translate into thousands of cases of dementia prevented. And this was only over 7 years. The benefit would likely grow over time.
The researchers were ready for objections.
Do we really know there were no important differences between the two groups? They checked. There was no difference in other preventive behaviors [2].
What about other interventions? Maybe something else used the same cutoff date. Again, no. Nothing else used that date [2].
And remember the earlier observational studies, where vaccination was linked to a range of health improvements? That didn't happen here. The two groups didn’t differ in other health outcomes. Only dementia rates diverged [2].
What’s next?
Still, one major question remained: Did the vaccine only prevent new cases of dementia? Or could it also slow progression in those already diagnosed?

The same research group ran a follow-up study using the same Wales population.
They found the vaccine reduced new diagnoses of mild cognitive impairment — an early stage of dementia — by 3.1% [4].
Even more remarkably, it appeared to slow the progression of dementia. Among people already diagnosed before the vaccine program began, the vaccinated had a 29.5 percentage point lower risk of dying from dementia over 9 years [4].
That’s a dramatic reduction.
It’s important to add some qualifications. The confidence intervals were wide, meaning the exact magnitude of the benefit is uncertain [4].
Also, this population was in a narrow age range — 79–80 years old. We don’t yet know how this translates to younger people [4].
Still, these studies give us compelling evidence that the shingles vaccine can slow or prevent dementia across the entire disease course [4].
And given that the vaccine is cheap, safe, and a one-time intervention, this is of huge significance for population health [4].
But there’s one lingering question.
The vaccine used in the Wales study was a live-attenuated version — Zostavax. This is being replaced by a newer recombinant vaccine — Shingrix [4].

Some research has found that broader health benefits — like immune system boosts — are stronger with live-virus vaccines [4].
Fortunately, a similar natural experiment in the U.S. took place after the introduction of Shingrix. The results were reassuring. The new recombinant vaccine was also associated with lower dementia risk — possibly even more than the live version [6].

One remaining puzzle is this: Is the apparent benefit due to protection from shingles specifically? Or is there a more general neuroprotective or immune-boosting mechanism at work?
Researchers believe both could be at play and are calling for more research to uncover the exact pathways [4].
We also need to clarify the ideal timing of vaccination for dementia prevention [4].
This is exciting — it could be a whole new strategy to prevent or treat dementia.
In the meantime
Other recent developments are giving us more potential strategies to reduce dementia risk.
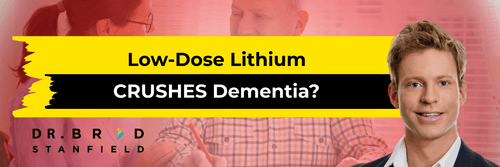
1. Lithium orotate
A study in mice found that lithium orotate almost completely prevented amyloid plaque and tau accumulation — both key hallmarks of Alzheimer’s [7].
2. Hearing aids
A systematic review of over 126,000 participants found that people with hearing loss who used hearing aids had a significantly lower risk of cognitive decline and dementia compared to those who didn’t [8].
3. Supplements
Here are four supplements supported by strong research:
- Multivitamin and mineral: A large 2-year trial showed improvements in cognition and memory. The benefit was equivalent to reducing brain aging by 2 years [9].
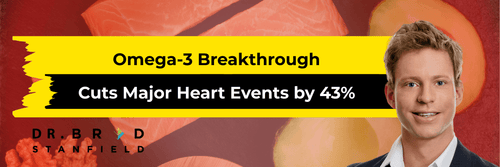
- Omega-3 fatty acids: A 2019 study showed omega-3s improved cognitive performance by 7.1% and reduced dementia symptoms by 22.3%. These effects were strongest when B vitamin levels were adequate [10].
- Creatine: Known for muscle performance, creatine also increases brain levels and improves memory — especially in older adults [11][12].
- TMG (Trimethylglycine): High homocysteine levels are a known risk factor for Alzheimer’s [13]. TMG lowers homocysteine levels [14], making it a promising preventive supplement.
I personally take a multivitamin & mineral, creatine, and TMG as part of MicroVitamin+ Powder. But just because I take a supplement doesn’t mean you need to.
References
1. https://pmc.ncbi.nlm.nih.gov/articles/PMC8597989/
2. https://pmc.ncbi.nlm.nih.gov/articles/PMC10246135/
3. https://pmc.ncbi.nlm.nih.gov/articles/PMC9006884/
4. https://www.cell.com/cell/fulltext/S0092-8674(25)01256-5
5. https://www.nejm.org/doi/10.1056/NEJMoa1501184?url_ver=Z39.88-2003
6. https://pmc.ncbi.nlm.nih.gov/articles/PMC11485228/
7. https://www.nature.com/articles/s41586-025-09335-x
8. https://www.thelancet.com/journals/lancet/article/PIIS0140-6736%2824%2901296-0/abstract
9. https://pmc.ncbi.nlm.nih.gov/articles/PMC11103094/
10. https://pubmed.ncbi.nlm.nih.gov/30958356/
11. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8912287/
12. https://academic.oup.com/nutritionreviews/advance-article/doi/10.1093/nutrit/nuac064/6671817